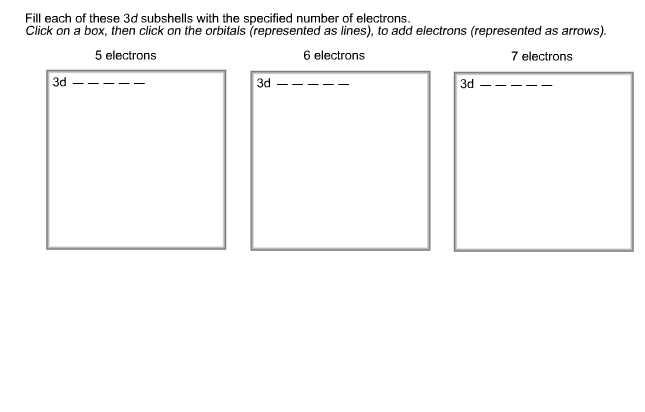
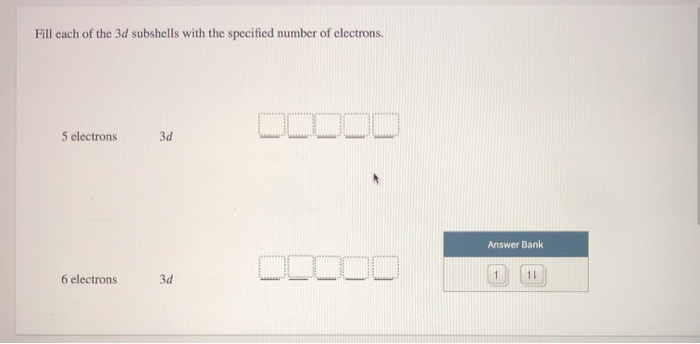
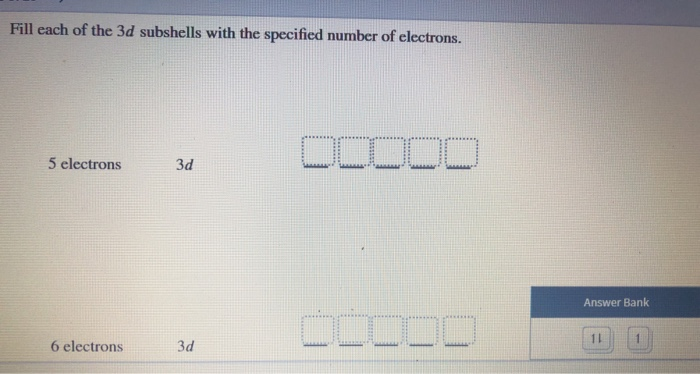
76+ pages fill each of these 3d subshells with 5 electrons 2.1mb. 7 electrons 5 electrons 6 electrons 3d 3d 3d. Electron must go into the second shell. FOUR possible subshells s p d f. Check also: each and learn more manual guide in fill each of these 3d subshells with 5 electrons 1 s-orbital and 3 p-orbitals.
7 electrons 5 electrons 6 electrons 3d 3d 3d. These are a group of orbitals that share the same principal quantum number azimuthal quantum numbers l.
Fill Each Of These 3d Subshells With The Specified Chegg
| Title: Fill Each Of These 3d Subshells With The Specified Chegg |
| Format: ePub Book |
| Number of Pages: 194 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: November 2019 |
| File Size: 1.2mb |
| Read Fill Each Of These 3d Subshells With The Specified Chegg |
 |
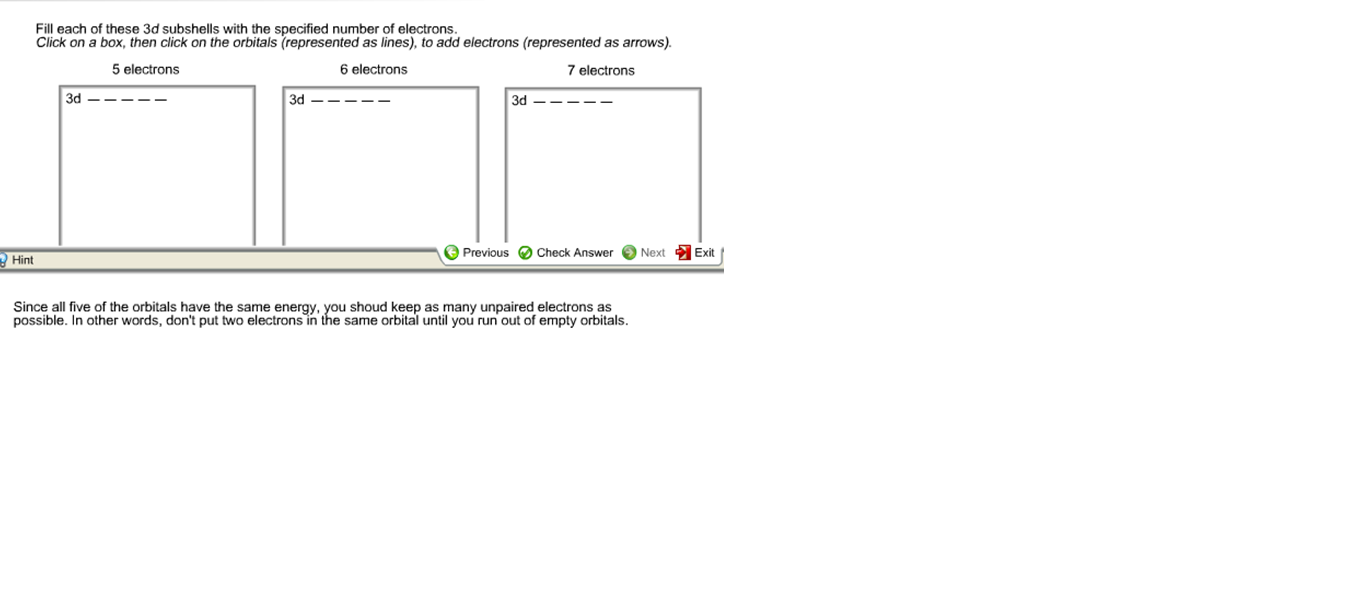
3d subshells with the specified number of electrons Click on a Show transcribed image text apling Learning Fill each of these 3d subshells with the specified number of electrons Click on a box then click on the orbitals represented as lines to add electrons represented as arrows.
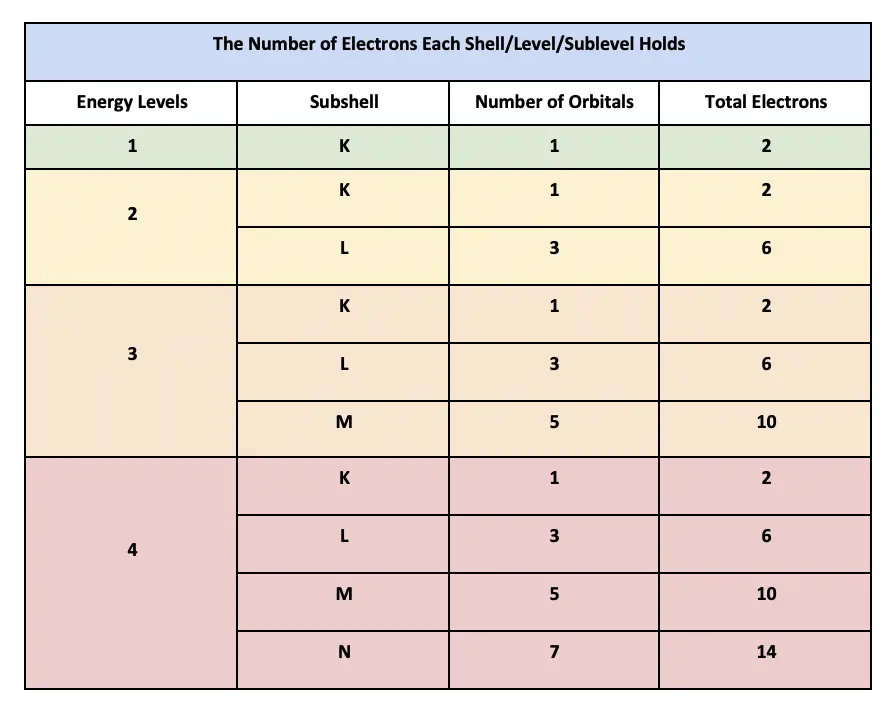
Also these first electrons have the same spin. The third shell has 3s 3p and 3d. And so on - Each orbital may at most contain TWO ELECTRONS. There are 4 subshells s p d and f each can hold different numbers of electrons. Be Z 4. TWO possible subshells s p 3rd shell.

Fill Each Of These 3d Subshells With The Specified Chegg
| Title: Fill Each Of These 3d Subshells With The Specified Chegg |
| Format: PDF |
| Number of Pages: 318 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: March 2020 |
| File Size: 1.35mb |
| Read Fill Each Of These 3d Subshells With The Specified Chegg |
 |
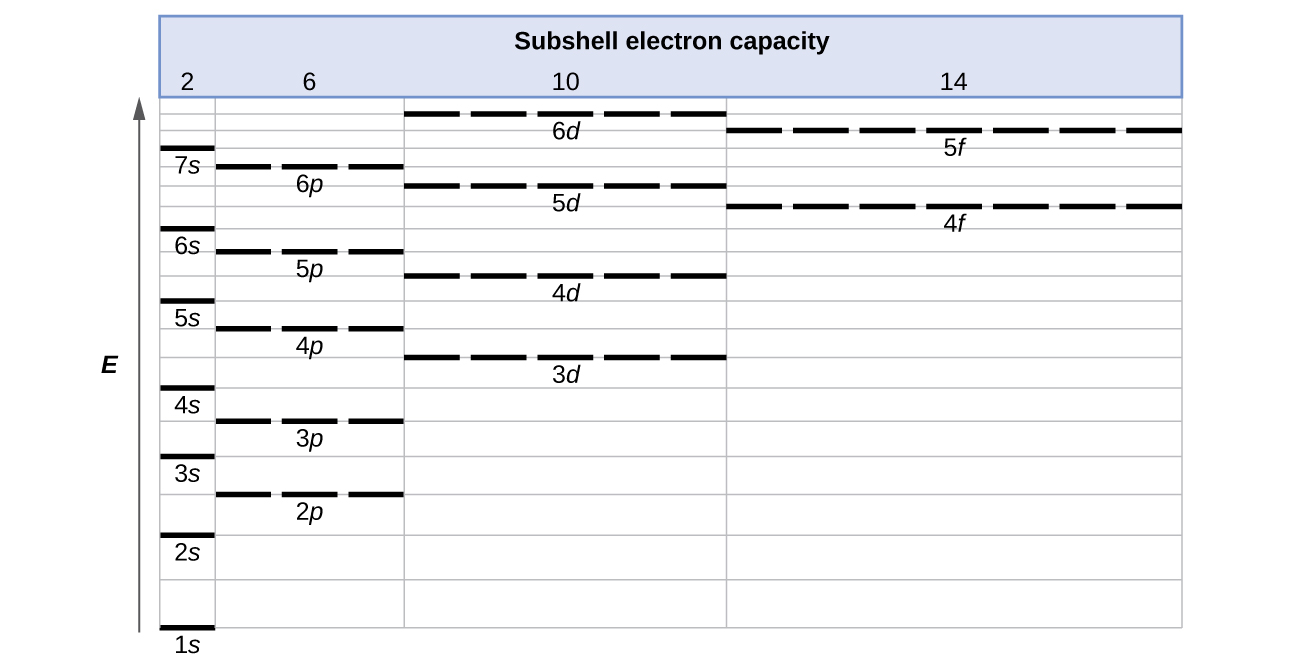
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts
| Title: 8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts |
| Format: PDF |
| Number of Pages: 207 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: March 2021 |
| File Size: 2.1mb |
| Read 8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts |
 |
Fill Each Of These 3d Subshells With The Specified Chegg
| Title: Fill Each Of These 3d Subshells With The Specified Chegg |
| Format: eBook |
| Number of Pages: 299 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: February 2018 |
| File Size: 1.35mb |
| Read Fill Each Of These 3d Subshells With The Specified Chegg |
 |
How Many Electrons Are In Each Shell Including 3p Orbitals
| Title: How Many Electrons Are In Each Shell Including 3p Orbitals |
| Format: ePub Book |
| Number of Pages: 227 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: May 2020 |
| File Size: 3.4mb |
| Read How Many Electrons Are In Each Shell Including 3p Orbitals |
 |

Electron Configurations Of The 3d Transition Metals Video Khan Academy
| Title: Electron Configurations Of The 3d Transition Metals Video Khan Academy |
| Format: PDF |
| Number of Pages: 298 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: January 2020 |
| File Size: 1.35mb |
| Read Electron Configurations Of The 3d Transition Metals Video Khan Academy |
 |

Oneclass Fill Each Of These 3d Subshells With The Specified Number Of Electrons 5 Electrons 6 Elect
| Title: Oneclass Fill Each Of These 3d Subshells With The Specified Number Of Electrons 5 Electrons 6 Elect |
| Format: ePub Book |
| Number of Pages: 137 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: October 2020 |
| File Size: 2.3mb |
| Read Oneclass Fill Each Of These 3d Subshells With The Specified Number Of Electrons 5 Electrons 6 Elect |
 |
Fill Each Of These 3d Subshells With The Specified Chegg
| Title: Fill Each Of These 3d Subshells With The Specified Chegg |
| Format: ePub Book |
| Number of Pages: 232 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: January 2019 |
| File Size: 810kb |
| Read Fill Each Of These 3d Subshells With The Specified Chegg |
 |

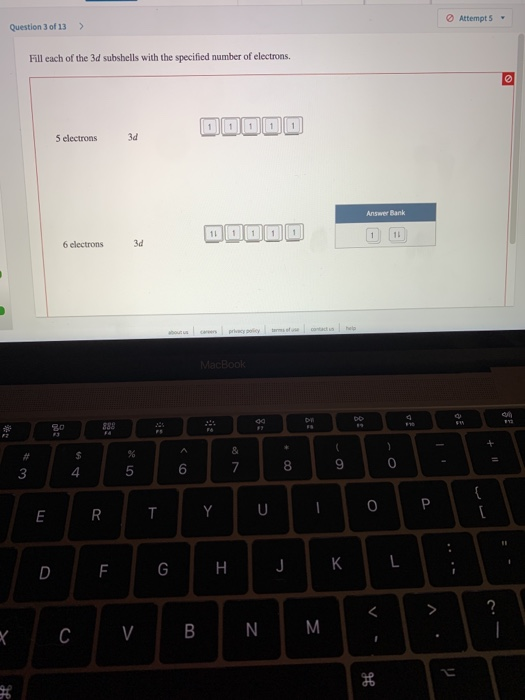
Question 3 Of 13 Attempts Fill Each Of The 3d Chegg
| Title: Question 3 Of 13 Attempts Fill Each Of The 3d Chegg |
| Format: eBook |
| Number of Pages: 342 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: November 2017 |
| File Size: 2.1mb |
| Read Question 3 Of 13 Attempts Fill Each Of The 3d Chegg |
 |
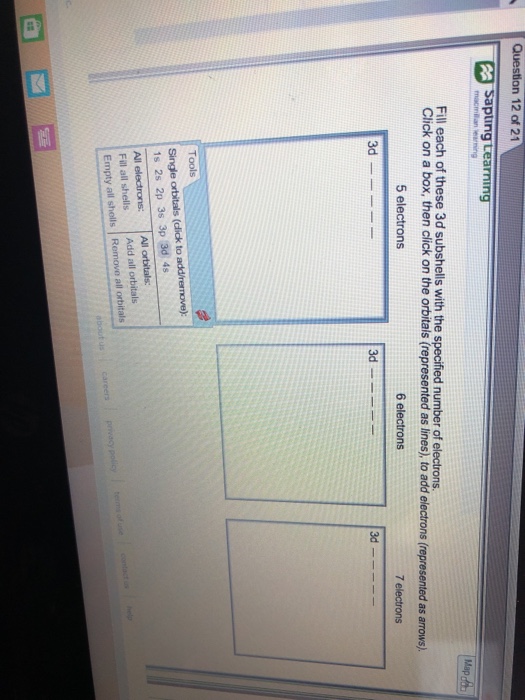
Question 12 Of 21 Sapiuing Learning Fill Each Of Chegg
| Title: Question 12 Of 21 Sapiuing Learning Fill Each Of Chegg |
| Format: PDF |
| Number of Pages: 246 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: March 2020 |
| File Size: 800kb |
| Read Question 12 Of 21 Sapiuing Learning Fill Each Of Chegg |
 |
Fill Each Of The 3d Subshells With The Specified Chegg
| Title: Fill Each Of The 3d Subshells With The Specified Chegg |
| Format: PDF |
| Number of Pages: 287 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: March 2017 |
| File Size: 1.4mb |
| Read Fill Each Of The 3d Subshells With The Specified Chegg |
 |
Fill Each Of The 3d Subshells With The Specified Chegg
| Title: Fill Each Of The 3d Subshells With The Specified Chegg |
| Format: PDF |
| Number of Pages: 200 pages Fill Each Of These 3d Subshells With 5 Electrons |
| Publication Date: September 2019 |
| File Size: 3.4mb |
| Read Fill Each Of The 3d Subshells With The Specified Chegg |
 |
Fill each of these 3d subshells with the specified number of electrons Clsck on a box then click on the orbitals represented as lines to add electrons represented as arrows. - Shells are numbered. ONE possible subshell s 2nd shell.
Here is all you need to know about fill each of these 3d subshells with 5 electrons In this video well discuss this in more depth and walk through all of the electron configurations for the 3d. Once a subshell becomes filled the subshell of the next higher energy starts to fill. The fifth shell has 5s 5p 5d and 5f and can theoretically hold more in the 5g subshell that is not. Fill each of the 3d subshells with the specified chegg question 3 of 13 attempts fill each of the 3d chegg question 12 of 21 sapiuing learning fill each of chegg electron configurations of the 3d transition metals video khan academy 8 3 electron configurations how electrons occupy orbitals chemistry libretexts how many electrons are in each shell including 3p orbitals 1s 2 2s 2 2p 2.
![Fill Each Of These 3d Subshells With 5 Electrons 32+ Pages Explanation [800kb] - Updated 2021 Fill Each Of These 3d Subshells With 5 Electrons 32+ Pages Explanation [800kb] - Updated 2021](https://media.cheggcdn.com/media%2Febf%2Febf4d886-c4f6-488c-9482-2ec24abc8855%2Fimage)